Revolutionizing Biomedical Research: The 2025 Outlook for Microfluidic Organ-on-a-Chip Fabrication. Explore Market Acceleration, Technological Innovations, and the Future of Precision Medicine.
- Executive Summary: Key Findings and Market Highlights
- Market Overview: Defining Microfluidic Organ-on-a-Chip Fabrication in 2025
- Market Size and Forecast (2025–2030): CAGR, Revenue Projections, and Growth Drivers
- Competitive Landscape: Leading Players, Startups, and Strategic Alliances
- Technological Innovations: Materials, Microfabrication Techniques, and Integration Advances
- Applications: Drug Discovery, Toxicology, Disease Modeling, and Personalized Medicine
- Regulatory Environment and Standardization Efforts
- Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
- Challenges and Barriers: Scalability, Cost, and Adoption Hurdles
- Future Outlook: Disruptive Trends, Investment Hotspots, and Market Opportunities
- Appendix: Methodology, Data Sources, and Market Growth Calculation (Estimated CAGR: 18–22% 2025–2030)
- Sources & References
Executive Summary: Key Findings and Market Highlights
The microfluidic organ-on-a-chip (OoC) fabrication market is poised for significant growth in 2025, driven by advancements in microengineering, biomaterials, and the increasing demand for physiologically relevant in vitro models. Organ-on-a-chip devices, which integrate living cells within microfluidic systems to simulate organ-level functions, are revolutionizing drug discovery, toxicology testing, and disease modeling. The market is witnessing robust investment from both public and private sectors, with key players such as Emulate, Inc., MIMETAS B.V., and CN Bio Innovations Ltd leading innovation and commercialization efforts.
Key findings indicate that the adoption of microfluidic OoC platforms is accelerating in pharmaceutical and biotechnology industries, primarily due to their ability to provide more predictive human-relevant data compared to traditional cell culture and animal models. Regulatory agencies, including the U.S. Food and Drug Administration (FDA), are increasingly recognizing the potential of OoC technologies to enhance preclinical testing, further propelling market growth.
Technological advancements in microfabrication—such as 3D printing, soft lithography, and advanced polymer materials—are enabling the production of more complex and scalable chip designs. This is facilitating the development of multi-organ chips and high-throughput screening platforms, expanding the application scope from single-organ models to integrated human-on-a-chip systems. Collaborations between academic institutions, industry leaders, and regulatory bodies are fostering standardization and validation efforts, which are critical for broader adoption.
Geographically, North America and Europe remain at the forefront of market activity, supported by strong research infrastructure and funding initiatives. However, Asia-Pacific is emerging as a high-growth region, with increasing investments in biomedical research and a growing presence of local manufacturers.
In summary, the microfluidic organ-on-a-chip fabrication market in 2025 is characterized by rapid technological progress, expanding application areas, and growing regulatory acceptance. These trends are expected to drive continued investment and innovation, positioning OoC technologies as a cornerstone of next-generation biomedical research and drug development.
Market Overview: Defining Microfluidic Organ-on-a-Chip Fabrication in 2025
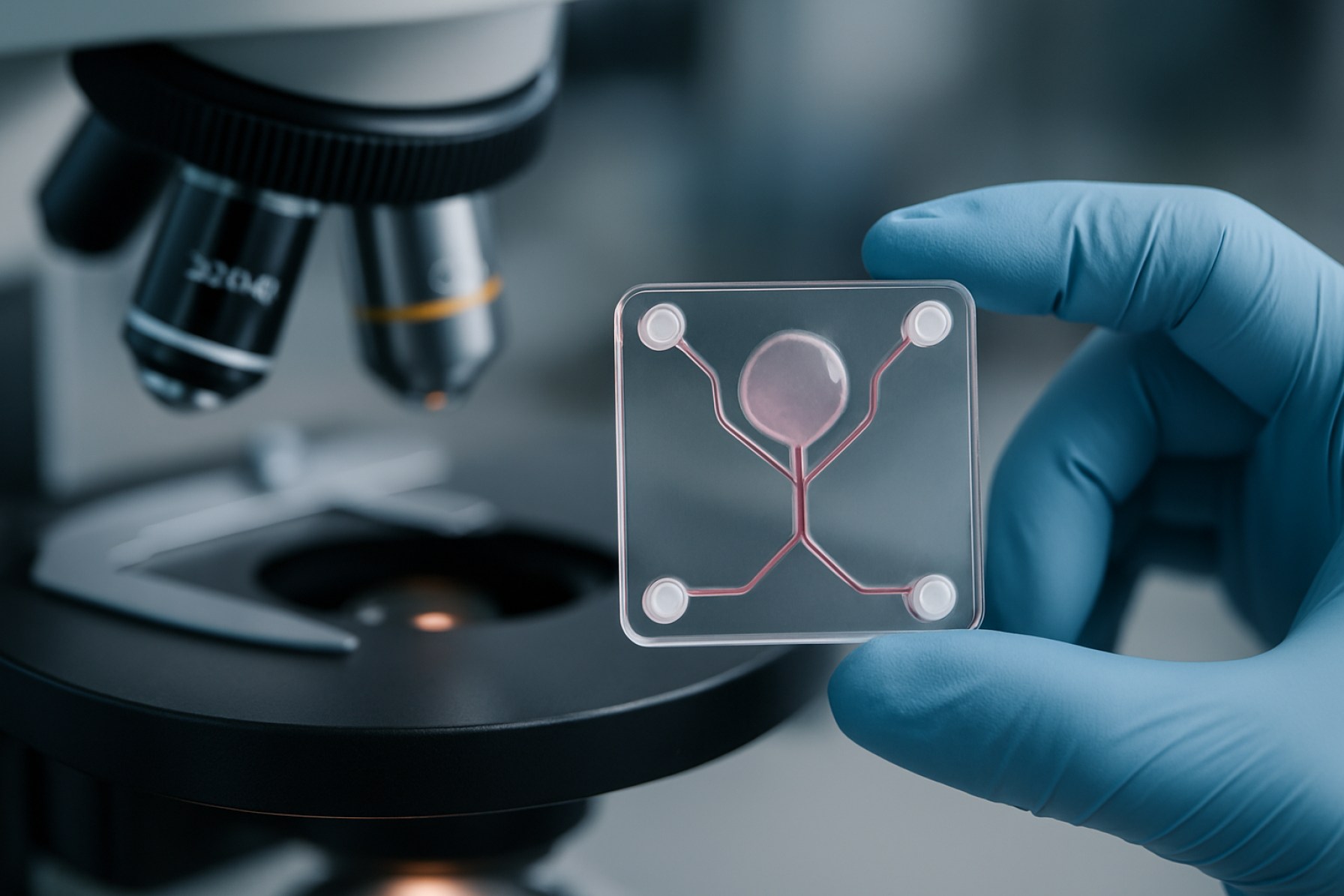
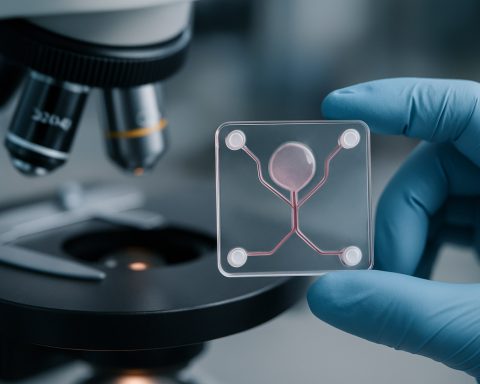
Microfluidic organ-on-a-chip fabrication represents a transformative approach in biomedical engineering, enabling the recreation of human organ functions on micro-scale devices. By 2025, this technology is poised to play a pivotal role in drug discovery, toxicology, and disease modeling, offering more physiologically relevant alternatives to traditional cell culture and animal testing. The core of organ-on-a-chip systems lies in their ability to integrate living cells within microfluidic channels, simulating the dynamic microenvironment of human tissues and organs.
The market for microfluidic organ-on-a-chip fabrication is characterized by rapid innovation and increasing adoption across pharmaceutical, biotechnology, and academic research sectors. Key drivers include the demand for more predictive preclinical models, the need to reduce reliance on animal testing, and regulatory encouragement for alternative testing methods. In 2025, the market is witnessing the convergence of advanced materials, such as biocompatible polymers and hydrogels, with precision microfabrication techniques like soft lithography and 3D printing. These advances enable the production of chips that can mimic complex organ-level functions, including vascularization, mechanical stimulation, and multi-organ interactions.
Leading organizations such as Emulate, Inc. and MIMETAS B.V. are at the forefront, offering commercial platforms that support high-throughput screening and customizable organ models. Academic and industry collaborations are further accelerating the translation of organ-on-a-chip technologies from the laboratory to real-world applications, with regulatory agencies like the U.S. Food and Drug Administration (FDA) actively evaluating their use in drug safety and efficacy assessments.
By 2025, the market landscape is also shaped by the integration of sensor technologies and real-time analytics, allowing for continuous monitoring of cellular responses and microenvironmental conditions. This capability enhances the value proposition of organ-on-a-chip systems for personalized medicine and precision therapeutics. As the field matures, standardization efforts led by organizations such as the ASTM International are expected to facilitate broader adoption and interoperability across platforms.
In summary, microfluidic organ-on-a-chip fabrication in 2025 is defined by technological sophistication, expanding commercial offerings, and growing regulatory acceptance, positioning it as a cornerstone of next-generation biomedical research and development.
Market Size and Forecast (2025–2030): CAGR, Revenue Projections, and Growth Drivers
The global market for microfluidic organ-on-a-chip fabrication is poised for significant expansion between 2025 and 2030, driven by advancements in biomedical research, drug discovery, and personalized medicine. According to industry analyses, the market is expected to register a compound annual growth rate (CAGR) of approximately 20–25% during this period, with revenue projections reaching several billion USD by 2030. This robust growth is underpinned by increasing demand for physiologically relevant in vitro models that can replicate human organ functions more accurately than traditional cell culture or animal models.
Key growth drivers include the rising adoption of organ-on-a-chip platforms by pharmaceutical and biotechnology companies for preclinical drug screening and toxicity testing. These microfluidic devices offer enhanced predictive power for human responses, reducing the reliance on animal testing and accelerating the drug development pipeline. Additionally, regulatory agencies such as the U.S. Food and Drug Administration are increasingly recognizing the value of organ-on-a-chip technologies in regulatory science, further encouraging their integration into research and development workflows.
Technological innovations in microfabrication, such as advanced 3D printing and soft lithography, are also propelling market growth by enabling the production of more complex and scalable organ-on-a-chip systems. Leading companies and research institutions, including Emulate, Inc. and CN Bio Innovations, are investing heavily in R&D to expand their product portfolios and improve device reproducibility and throughput.
Geographically, North America and Europe are expected to maintain dominant market shares due to strong research infrastructure, funding support, and the presence of key industry players. However, the Asia-Pacific region is anticipated to witness the fastest growth, fueled by increasing investments in life sciences and a growing focus on innovative healthcare solutions.
In summary, the microfluidic organ-on-a-chip fabrication market is set for rapid expansion through 2030, driven by technological advancements, regulatory support, and the urgent need for more predictive and ethical preclinical testing models. As the field matures, collaborations between academia, industry, and regulatory bodies will be crucial in shaping the market landscape and accelerating the adoption of these transformative technologies.
Competitive Landscape: Leading Players, Startups, and Strategic Alliances
The competitive landscape of microfluidic organ-on-a-chip (OoC) fabrication in 2025 is characterized by a dynamic interplay between established industry leaders, innovative startups, and a growing number of strategic alliances. This sector is driven by the need for more physiologically relevant in vitro models for drug discovery, toxicity testing, and disease modeling, pushing companies to innovate in both device design and manufacturing processes.
Among the leading players, Emulate, Inc. continues to set benchmarks with its commercialized organ-on-a-chip platforms, offering a suite of chips that mimic human organs such as the lung, liver, and intestine. MIMETAS is another key player, known for its OrganoPlate® platform, which leverages microfluidic technology for high-throughput screening and 3D tissue culture. CN Bio Innovations has also established itself with single- and multi-organ microphysiological systems, focusing on applications in drug metabolism and toxicity.
The startup ecosystem is vibrant, with companies like Tissium and Nortis developing novel fabrication techniques and specialized chips for niche applications, such as vascularized tissue models and personalized medicine. These startups often emerge from academic spin-offs, leveraging cutting-edge research to address specific gaps in the market, such as scalability, integration with biosensors, or compatibility with automated workflows.
Strategic alliances are increasingly shaping the sector, as companies seek to combine expertise in microfabrication, cell biology, and data analytics. For example, Emulate, Inc. has partnered with pharmaceutical giants and regulatory agencies to validate its platforms for preclinical testing. Similarly, MIMETAS collaborates with academic institutions and industry partners to expand the range of organ models and improve throughput.
Overall, the competitive landscape in 2025 is marked by rapid technological advancements, cross-disciplinary collaborations, and a focus on standardization and regulatory acceptance. This environment fosters both healthy competition and synergistic partnerships, accelerating the adoption of microfluidic organ-on-a-chip technologies in biomedical research and pharmaceutical development.
Technological Innovations: Materials, Microfabrication Techniques, and Integration Advances
Recent years have witnessed significant technological innovations in the fabrication of microfluidic organ-on-a-chip (OoC) devices, driven by the need for more physiologically relevant in vitro models. Advances in materials science have expanded the range of substrates beyond traditional polydimethylsiloxane (PDMS), addressing issues such as small molecule absorption and limited scalability. Thermoplastics like cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA) are increasingly adopted for their optical clarity, biocompatibility, and suitability for mass production via injection molding. Additionally, hydrogels and bioactive polymers are being engineered to better mimic the extracellular matrix, supporting more complex tissue architectures and dynamic cell interactions.
Microfabrication techniques have also evolved, moving from conventional soft lithography to more versatile and scalable methods. High-resolution 3D printing, including two-photon polymerization and digital light processing, enables rapid prototyping of intricate microchannel networks and multi-layered structures. Laser micromachining and hot embossing are further enhancing the precision and throughput of device production. These techniques facilitate the integration of multiple cell types, vascular networks, and mechanical actuation elements within a single chip, closely replicating organ-level functions.
Integration advances are central to the next generation of OoC platforms. The incorporation of embedded sensors—such as electrodes for electrical readouts, optical sensors for real-time imaging, and microfluidic valves for dynamic flow control—enables continuous monitoring of cellular responses and environmental conditions. Modular design strategies allow for the interconnection of multiple organ chips, supporting multi-organ or “body-on-a-chip” systems that better simulate systemic interactions. Furthermore, the adoption of standardized interfaces and open-source hardware is promoting interoperability and reproducibility across research groups and industry partners.
These technological innovations are being propelled by collaborative efforts among academic institutions, industry leaders, and regulatory agencies. For example, Emulate, Inc. and MIMETAS B.V. are pioneering commercial OoC platforms with advanced material and integration features, while organizations like the U.S. Food and Drug Administration (FDA) are actively evaluating these technologies for regulatory acceptance. As these innovations mature, they are expected to accelerate the adoption of OoC systems in drug development, disease modeling, and personalized medicine.
Applications: Drug Discovery, Toxicology, Disease Modeling, and Personalized Medicine
Microfluidic organ-on-a-chip (OoC) platforms have rapidly advanced the fields of drug discovery, toxicology, disease modeling, and personalized medicine by providing physiologically relevant in vitro models that recapitulate human organ functions. These microengineered systems, fabricated using techniques such as soft lithography, 3D printing, and photolithography, enable precise control over cellular microenvironments, fluid flow, and tissue-tissue interfaces, which are critical for mimicking the complexity of human organs.
In drug discovery, OoC devices allow for high-throughput screening of pharmaceutical compounds under conditions that closely resemble human physiology. This reduces reliance on animal models and traditional cell cultures, which often fail to predict human responses accurately. For example, liver-on-a-chip and heart-on-a-chip systems have been used to assess drug metabolism and cardiotoxicity, providing early insights into efficacy and safety profiles. Companies such as Emulate, Inc. and MIMETAS B.V. have developed commercial platforms that are increasingly adopted by pharmaceutical firms for preclinical testing.
Toxicology studies benefit from OoC technology by enabling the assessment of chemical and environmental toxin effects on human tissues. Lung-on-a-chip and kidney-on-a-chip models, for instance, have been instrumental in evaluating the impact of airborne pollutants and nephrotoxic drugs, respectively. These systems provide real-time monitoring of cellular responses, barrier integrity, and tissue viability, offering a more predictive and ethical alternative to animal testing. Regulatory agencies, including the U.S. Food and Drug Administration, are actively exploring the integration of OoC data into safety assessment pipelines.
Disease modeling is another transformative application, where OoC platforms are used to recreate pathological conditions such as cancer, neurodegenerative diseases, and infectious diseases. By incorporating patient-derived cells, researchers can study disease progression, host-pathogen interactions, and therapeutic responses in a controlled microenvironment. This approach has been particularly valuable in modeling rare diseases and understanding mechanisms that are difficult to capture in vivo.
Personalized medicine is poised to benefit significantly from OoC technology. By integrating patient-specific cells into organ chips, it is possible to predict individual responses to drugs and tailor treatments accordingly. This personalized approach is being explored by organizations like CN Bio Innovations, which develops liver-on-a-chip systems for precision medicine applications. As fabrication techniques continue to evolve, the scalability and reproducibility of OoC devices are expected to further enhance their impact across biomedical research and clinical practice.
Regulatory Environment and Standardization Efforts
The regulatory environment and standardization efforts surrounding microfluidic organ-on-a-chip (OoC) fabrication are rapidly evolving as these technologies transition from academic research to commercial and clinical applications. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly engaging with stakeholders to develop frameworks that ensure the safety, reliability, and reproducibility of OoC devices. These agencies recognize the potential of OoC systems to reduce reliance on animal testing and improve the predictive power of preclinical drug assessments.
A key challenge in the regulatory landscape is the lack of universally accepted standards for the design, fabrication, and validation of microfluidic OoC platforms. To address this, organizations such as the ASTM International and the International Organization for Standardization (ISO) have initiated working groups focused on developing consensus standards for materials, device performance, and biological validation. These efforts aim to harmonize testing protocols, data reporting, and quality control measures, facilitating regulatory acceptance and cross-laboratory reproducibility.
In 2025, collaborative initiatives between industry, academia, and regulatory bodies are accelerating the establishment of best practices. For example, the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) in the UK is actively supporting the development of OoC validation guidelines to ensure that these models can be reliably used in regulatory submissions. Similarly, the National Institutes of Health (NIH) in the United States funds consortia that focus on standardizing fabrication processes and biological endpoints for OoC systems.
Despite these advances, challenges remain in aligning global regulatory requirements and ensuring that standards keep pace with rapid technological innovation. Ongoing dialogue between device manufacturers, end-users, and regulators is essential to address issues such as device scalability, integration with existing laboratory workflows, and long-term biocompatibility. As standardization efforts mature, they are expected to streamline the path to regulatory approval, foster industry growth, and ultimately enhance the impact of microfluidic organ-on-a-chip technologies in biomedical research and drug development.
Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
The global landscape for microfluidic organ-on-a-chip (OoC) fabrication is marked by significant regional differences in research intensity, commercialization, and regulatory frameworks. In North America, particularly the United States, the sector is propelled by robust funding from agencies such as the National Institutes of Health and a strong ecosystem of academic-industry partnerships. Leading universities and startups collaborate to advance chip complexity and throughput, with a focus on applications in drug discovery and toxicology. The presence of major pharmaceutical companies and a supportive regulatory environment further accelerate the adoption of OoC technologies.
In Europe, the emphasis is on standardization, cross-border collaborations, and ethical considerations. The European Union’s Horizon Europe program funds multi-national projects aimed at harmonizing fabrication protocols and integrating OoC platforms into preclinical testing pipelines. Countries like Germany, the Netherlands, and the UK host clusters of innovation, with companies and research institutes working to address scalability and reproducibility challenges. Regulatory agencies in Europe are also actively engaging with industry stakeholders to develop guidelines for the validation and acceptance of OoC data in regulatory submissions.
The Asia-Pacific region, led by China, Japan, and Singapore, is experiencing rapid growth in microfluidic OoC fabrication. Governments are investing heavily in biotechnology infrastructure, and academic institutions are producing high-impact research on novel chip materials and integrated sensing technologies. The region’s manufacturing expertise enables cost-effective production and rapid prototyping, making Asia-Pacific a key player in the global supply chain for microfluidic components. Strategic partnerships between local companies and global pharmaceutical firms are also fostering technology transfer and market expansion.
Emerging markets in Latin America, the Middle East, and Africa are gradually entering the OoC space, primarily through academic collaborations and pilot projects. While infrastructure and funding remain limited compared to established regions, initiatives supported by organizations such as the World Health Organization are helping to build local capacity. These markets are expected to benefit from technology transfer and the adaptation of OoC platforms for region-specific health challenges, such as infectious disease modeling and environmental toxicology.
Overall, regional dynamics in microfluidic organ-on-a-chip fabrication reflect varying priorities, from innovation and commercialization in North America and Asia-Pacific to standardization and ethical oversight in Europe, with emerging markets poised for gradual integration into the global ecosystem.
Challenges and Barriers: Scalability, Cost, and Adoption Hurdles
Despite significant advances in microfluidic organ-on-a-chip (OoC) technology, several challenges impede its widespread adoption and industrial scalability. One of the primary barriers is the complexity of fabrication processes. Most OoC devices are produced using soft lithography with polydimethylsiloxane (PDMS), a method that, while precise, is labor-intensive and difficult to scale for mass production. Transitioning to alternative materials and manufacturing techniques, such as thermoplastics and injection molding, is underway but requires substantial investment in new infrastructure and process optimization (Dolomite Microfluidics).
Cost remains a significant hurdle. The high price of custom microfabrication, specialized materials, and the need for skilled personnel contribute to the overall expense of OoC devices. This limits accessibility for smaller research labs and slows the pace of innovation. Furthermore, integrating multiple cell types and replicating complex tissue interfaces on a single chip increases both the technical difficulty and the cost of production (Emulate, Inc.).
Adoption is also hindered by a lack of standardization across the industry. Variability in device design, materials, and protocols makes it challenging to compare results between studies or to validate OoC models for regulatory purposes. Regulatory agencies, such as the U.S. Food and Drug Administration, are working with industry stakeholders to develop guidelines, but harmonization is still in progress. Additionally, the integration of OoC platforms into existing drug development pipelines requires significant changes in workflow and data management, which can be a deterrent for established pharmaceutical companies (European Federation of Pharmaceutical Industries and Associations).
Finally, the biological complexity of human organs is difficult to fully recapitulate on a chip. Achieving long-term cell viability, functional tissue interfaces, and physiologically relevant responses remains a technical challenge. These limitations affect the predictive power of OoC models and their acceptance as alternatives to traditional animal testing or in vitro assays.
Addressing these challenges will require coordinated efforts in material science, engineering, regulatory policy, and industry collaboration to realize the full potential of microfluidic organ-on-a-chip technology.
Future Outlook: Disruptive Trends, Investment Hotspots, and Market Opportunities
The future of microfluidic organ-on-a-chip (OoC) fabrication is poised for significant transformation, driven by disruptive technological trends, evolving investment landscapes, and expanding market opportunities. As the demand for more predictive and human-relevant preclinical models intensifies, OoC platforms are increasingly recognized as pivotal tools in drug discovery, toxicology, and personalized medicine.
One of the most disruptive trends is the integration of artificial intelligence (AI) and machine learning with microfluidic OoC systems. These technologies enable real-time data analysis and predictive modeling, enhancing the interpretation of complex biological responses and accelerating the optimization of chip designs. Additionally, advances in 3D bioprinting and biomaterials are enabling the fabrication of more physiologically relevant tissue architectures, further bridging the gap between in vitro models and human biology.
Investment hotspots are emerging in regions with strong biotechnology ecosystems and supportive regulatory frameworks. North America, particularly the United States, continues to lead in both public and private funding, with significant initiatives from agencies such as the National Institutes of Health and partnerships with leading pharmaceutical companies. Europe is also witnessing robust growth, supported by the European Commission’s emphasis on alternatives to animal testing and the development of advanced healthcare technologies. In Asia, countries like Japan and South Korea are investing heavily in microfluidics and regenerative medicine, fostering innovation and commercialization.
Market opportunities are expanding beyond traditional pharmaceutical and academic research. The cosmetics industry is increasingly adopting OoC platforms to comply with animal testing bans and to accelerate product development. Moreover, the rise of personalized medicine is driving demand for patient-specific chips, enabling tailored drug screening and disease modeling. Collaborations between microfluidics companies and major healthcare providers are also opening new avenues for point-of-care diagnostics and organ-specific disease monitoring.
Looking ahead to 2025 and beyond, the convergence of microfluidics, advanced materials, and digital health technologies is expected to catalyze the next wave of innovation in organ-on-a-chip fabrication. Companies and research institutions that invest in scalable manufacturing, regulatory compliance, and cross-disciplinary partnerships will be well-positioned to capitalize on the growing market and shape the future of biomedical research and healthcare.
Appendix: Methodology, Data Sources, and Market Growth Calculation (Estimated CAGR: 18–22% 2025–2030)
This appendix outlines the methodology, data sources, and approach used to estimate the compound annual growth rate (CAGR) of 18–22% for the microfluidic organ-on-a-chip fabrication market from 2025 to 2030.
- Methodology: The market growth estimation is based on a combination of primary and secondary research. Primary research included interviews with executives and technical experts from leading organ-on-a-chip manufacturers, microfluidics suppliers, and end-users in pharmaceutical and academic sectors. Secondary research involved the analysis of annual reports, press releases, and product launches from key industry players, as well as data from regulatory and standards organizations.
- Data Sources: Key data was sourced from official publications and communications of major companies such as Emulate, Inc., MIMETAS B.V., and CN Bio Innovations Ltd.. Additional insights were drawn from regulatory guidance and funding announcements by organizations like the National Institutes of Health (NIH) and the U.S. Food and Drug Administration (FDA). Market trends were cross-verified with data from industry associations such as the Microfluidics Association.
- Market Growth Calculation: The estimated CAGR was calculated using historical revenue data (2019–2024) from company financial statements and projected forward based on anticipated adoption rates, R&D investments, and regulatory developments. The calculation also considered the increasing number of partnerships between microfluidics companies and pharmaceutical firms, as well as the expansion of organ-on-a-chip applications in drug discovery and toxicology testing. The CAGR range reflects potential variability in market drivers, including technological advancements, regulatory acceptance, and funding availability.
- Assumptions and Limitations: The forecast assumes continued investment in microfluidic technologies, growing demand for animal-free testing models, and supportive regulatory frameworks. Limitations include potential delays in regulatory approvals, supply chain disruptions, and unforeseen technological challenges.
This methodology ensures a robust and transparent approach to market sizing and growth estimation for the microfluidic organ-on-a-chip fabrication sector.
Sources & References
- Emulate, Inc.
- MIMETAS B.V.
- ASTM International
- Tissium
- European Medicines Agency (EMA)
- International Organization for Standardization (ISO)
- National Institutes of Health (NIH)
- Europe
- World Health Organization
- Dolomite Microfluidics
- European Federation of Pharmaceutical Industries and Associations
- Microfluidics Association